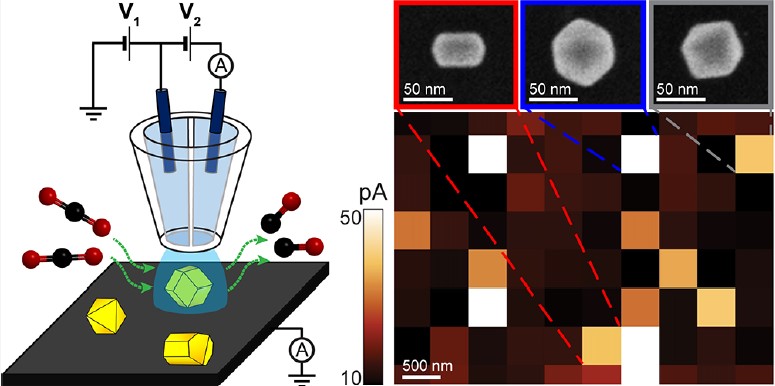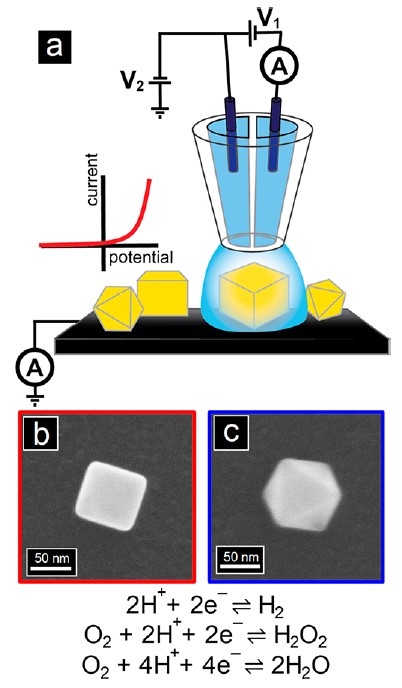
The Baker group studied single-entity electrochemistry in the collaboration with Ye group (Indiana University Bloomington) to study and characterize electrocatalytic activity of single nanocrystals by utilizing scanning electrochemical cell microscopy (SECCM) and correlative microscopy. The methodology framework for SEE suggested here could inform future studies of complex electrocatalytic processes through correlative single-entity and macroscale measurement techniques.
In the first study, electroreduction reactions (i.e., the hydrogen evolution reaction (HER)
and the oxygen reduction reaction) at individual, faceted Au nanocubes (NCs) and nano-octahedra
(ODs) expressing predominantly {100} and {111} crystal planes on the surface, respectively,
were studied by nanoscale voltammetric mapping of SECCM. Cyclic voltammograms were collected at individual nanoparticles (NPs) with SECCM and correlated with particle morphology imaged by electron microscopy. Nanoscale measurements from a statistically informative set of individual NPs revealed that Au NCs have superior HER electrocatalytic activity compared to that of Au ODs, in good agreement with macroscale cyclic voltammetry measurements. Au NCs exhibited more particle-to-particle variation in catalytic activity compared to that with Au ODs.
In a second study, the performance of nanocrystal catalysts for the CO2 reduction reaction (CO2RR) was measured by the single-entity electrochemistry methodology with comparison to classic macroscale measurement for nanocrystal ensembles, which returns an averaged system-level response of complex catalyst-modified electrodes with each nanocrystal likely contributing a different amount.
Measurements at single nanocrystals, taken in the context of statistical analysis of a population, and comparison to macroscale measurements are necessary to untangle the complexity of the ever-present heterogeneity in nanocrystal catalysts, achieve true structure−property correlation, and potentially identify nanocrystals with outlier performance.
Here, environment-controlled SECCM was employed to isolate and investigate the electrocatalytic CO2RR response of individual facet-defined gold nanocrystals. Using correlative microscopy approaches, it was conclusively demonstrated that {110}-terminated gold rhombohedra possess superior activity and selectivity for CO2RR compared with {111}-terminated octahedra and high-index {310}-terminated truncated ditetragonal prisms,
especially at low overpotentials where electrode kinetics is anticipated to dominate the current response.